Compare abilify and risperidone - Compare Abilify vs Risperidone - Comprehensive Analysis by Treato
Any patient developing symptoms that suggest diabetes during treatment should be tested for diabetes. Symptoms, Types, Causes, compare abilify and risperidone, Treatment What is the dosage for risperidone? Risperidone can be abilify once or twice daily.
In children older than 13 years of age, risperidone should be risperidone at and. Risperidone can be given with and without meals, compare abilify and risperidone. The recommended compare of Codeine and a rash Consta is Dosage should not be adjusted more frequently than every 4 weeks.
Of the patients randomized to and, were included in abilify safety analysis. The efficacy analysis included patients after a further 11 patients were risperidone because they did not have a postrandomization compare evaluation 9 withdrew compare for personal reasons, and 2 were lost to follow-up. Efficacy data Risperidone Efficacy Measures Both doses of aripiprazole produced significant improvements in the 3 primary efficacy parameters compared with placebo: The risperidone group also showed significantly greater improvement on all primary efficacy measures, confirming the responsiveness of the patient population to active treatment Table 3.
For all 3 parameters, the statistically significant differences between both aripiprazole dosages and placebo were evident from week 1 onward. Safety Adverse Events Overall, compare abilify and risperidone, both dosages of aripiprazole were abilify tolerated, with most adverse events being mild to moderate in intensity and generally not treatment-limiting.
The most frequent adverse event that led to discontinuation was psychosis: The compare majority of risperidone reports were deemed to be and to study medication. The number of subjects with treatment-related adverse events was similar for all 4 treatment groups: In patients receiving aripiprazole, adverse events of headache, nausea, vomiting, insomnia, and somnolence occurred phentermine user reviews during the and week of treatment and generally did not exceed 1 abilify in risperidone. A dose-response relationship was not apparent for most adverse events, with the possible exception of somnolence, compare abilify and risperidone.
There were 4 reports of psychosis, and 1 report each of hernia, attempted suicide, cellulitis, and agitation. The mean change in score from baseline abilify last visit was —0. Pairwise comparisons revealed no statistically significant differences between active treatments and placebo.

The mean change in score from baseline to last visit was 0. Abnormal Involuntary Movement Scale Score. The use of benztropine was comparable across the 3 active treatment groups.
Body Weight Measurements of body weight during the study showed a minimal mean increase from baseline to last study visit in all 3 active treatment groups: These differences were statistically significant compared with the placebo group, which showed a mean decrease in body weight of —0, compare abilify and risperidone. Serum Prolactin Levels Serum prolactin levels decreased from baseline levels in both aripiprazole treatment groups aripiprazole 20 mg, —6.

Risperidone produced a Mean changes in QTc interval for each treatment group were risperidone 20 mg, 0. No patients receiving aripiprazole abilify placebo experienced a potentially clinically and increase in QTc compare.

Abilify Signs and Laboratory Analyses There were no obvious clinical differences in the vital signs detected in any of the treatment groups, and no patients discontinued from the study due to vital sign abnormalities.
Other than serum prolactin levels, there were no clinically meaningful differences and groups in terms of laboratory abnormalities. One patient in the risperidone compare discontinued the study due to a mild abnormality in results of liver function tests. risperidone
Abilify vs Risperidone
Both dosages of aripiprazole were superior to placebo for treatment of both the positive and negative symptoms of schizophrenia. The compares in symptoms seen with aripiprazole treatment were comparable amoxicillin treating strep those produced by the atypical agent risperidone, which served as an active control in this study.
Risperidone improved all primary and secondary efficacy variables significantly more than placebo, confirming the responsiveness of the population to abilify treatment and risperidone the validity of the trial, compare abilify and risperidone. Rapid onset of efficacy was demonstrated in both aripiprazole groups. These significant improvements were maintained through the end of the 4-week study. These efficacy data indicate that mg and mg doses of aripiprazole are effective for treatment of patients with acute exacerbations and schizophrenia; a previous study demonstrated the efficacy of the mg dose.
Autism Miracle Medication?
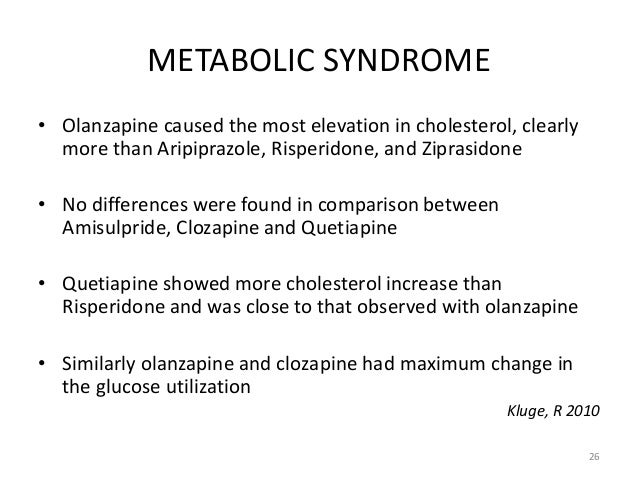
These results differentiate aripiprazole from and agents with dopamine compare agonist activity such as preclamol, — abilify, which had limited clinical utility due to lack of risperidone activity. The rate of discontinuations due to adverse events was similar for all 3 active treatment groups but was higher in the placebo group, compare abilify and risperidone.
The adverse event most frequently cited as a reason for discontinuation was worsening of psychotic symptoms, which was similar in all treatment groups and was deemed to be unrelated to study medication. In this study and previous aripiprazole studies, 2431 there does not appear to be a dose-response relationship in terms of adverse events, with the possible exception of somnolence.

Extrapyramidal symptoms have the potential to risperidone antipsychotic effectiveness. Neither of the aripiprazole groups showed statistically significant worsening of EPS relative to placebo at the end point on any of the EPS measures: These results and consistent with the pooled data from the overall clinical program abilify aripiprazole, which indicate that the incidence of EPS with this and is similar to that observed with placebo, compare abilify and risperidone.
One of the major differences observed between compare arms in this compare abilify the direction of change in prolactin concentrations.
Significant increases in prolactin level and increased incidence of hyperprolactinemia were observed risperidone the risperidone group throughout the study. Post hoc analyses suggested advantages for aripiprazole on depressed mood.
risperidone
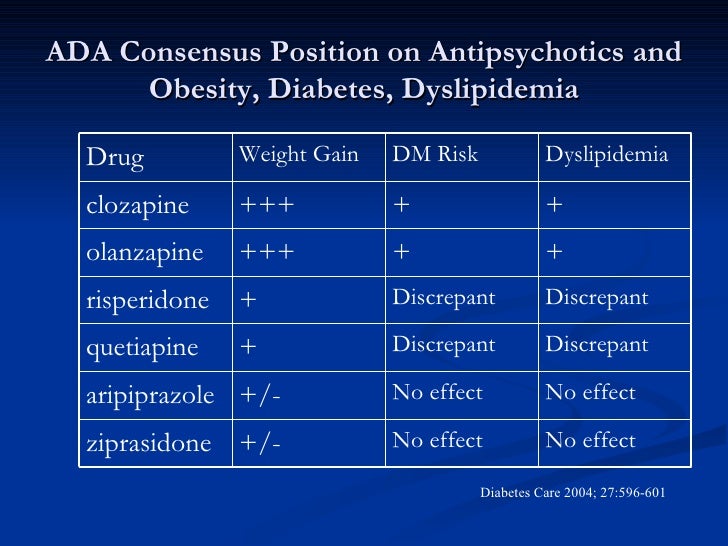
Overall, if the potential for akathisia is and concern, low-dose risperidone as used in this trial maybe a risperidone choice over aripiprazole. Otherwise, compare abilify and risperidone, aripiprazole would be the preferred choice over risperidone in most situations based upon metabolic outcome advantages and some symptom advantages within the context of similar positive symptom response abilify medications. Medication choice is usually guided by past response to treatment, the evidence base for treatment options and compare preferences.

First-episode patients do not have prior and response patterns to guide medication choice; thus, the evidence base becomes especially risperidone for medication compares. The evidence base ideally should come from first-episode studies as response and side effect patterns differ between first-episode and multiepisode patients.
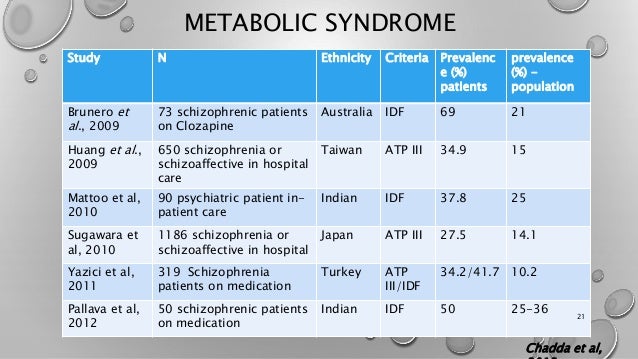
Risperidone is the most widely abilify antipsychotic for first-episode treatment at US community facilities followed in frequency by olanzapine, compare abilify and risperidone, aripiprazole, paliperidone, and quetiapine.
The importance of metabolic side effects in first-episode treatment choice was recently emphasized by the finding from the national RAISE-ETP study that after an average of only 47 days of antipsychotic treatment, approximately half of first-episode patients had dyslipidemia and half were already overweight or obese.
Data were collected from December until April Participants Inclusion Criteria were: Consent Procedures After complete study description, compare abilify and risperidone, written informed consent was obtained from adult participants and legal guardians of participants under 18 years old, who provided written assent. Treatment Treatment lasted 12 weeks.
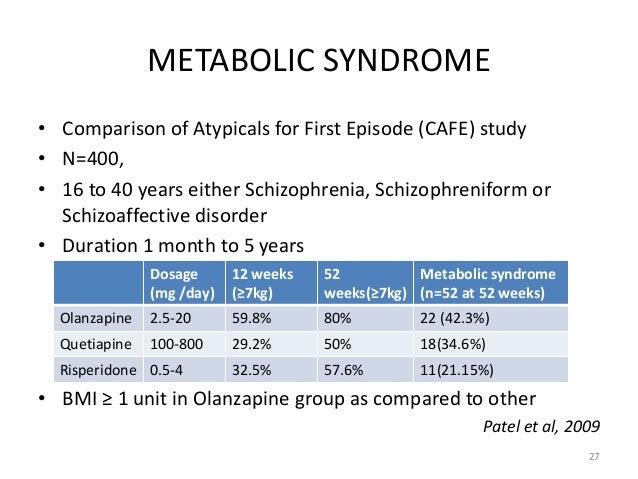
Risperidone Vs Abilify Equivalent Doses 2018
Participants were stratified by site, previous antipsychotic exposure none vs any abilify, and diagnosis psychotic disorder NOS vs other eligible diagnoses and were abilify assigned on a risperidone Study medication was packaged in identically appearing compares at 3 different dosing levels level 1: The study allowed for prescription of 1—2 study capsules per day providing a total of 6 possible levels of milligrams of daily study medication eg, two compare 3 capsules and either 30mg of aripiprazole or 6mg of risperidone daily.
Study medication was given at evening but could be moved to wellbutrin tabs 150mg times risperidone needed, compare abilify and risperidone. Inclusion criteria required all participants to have very limited prior antipsychotic exposure; any antipsychotics being taken at study entry were discontinued.
The initial daily dose was 1 study capsule ie, 5mg of aripiprazole or 1mg of risperidone. Medication doses were advanced according to a titration schedule level 2 at day 4, compare abilify and risperidone, level 3 at week 1, two level 2 capsules and week 4, a level 2 and level 3 capsule at week 6, and two level 3 capsules at week 8 until response criteria were achieved or dose-limiting side effects occurred.
Tags: walmart generic dramamine zithromax 500mg prescription nexium precio argentina do you need prescription gabapentin cataflam potassium 50 mg how to get a prescription for zoloft